- Emission
- Installation of CEMS equipment
- CEMS = Continuous Emission Monitoring System. In such systems, the probe must always be placed after the last step of flue gas treatment before the stack or in the stack. This is done to ensure that the measured values reflect the actual emission to the atmosphere. Some components such as CO, O2, NO and CO2 may be measured earlier in the process, provided that they are not influenced by for instance a flue gas condenser or wet scrubber. The probe must be placed on a straight, homogeneous length of the duct, 5 x duct diameter after a bend, restriction, expansion or the like as well as min. 1 x duct diameter before a bend, restriction, expansion or the like.
- How do I gather and report my emission data?
- In case of demands from the authorities on gathering and reporting of emission data, this may be solved with FLSmidth's ReportLoq, an environmental reporting system adapted to the individual customer's needs and the authorities' demands. ReportLoq may be delivered as a cloud-based or a stand-alone solution. An introduction video may be found here:
https://www.reportloq.com - Customer area of responsibility
- Why must my dust monitor be calibrated gravimetrically?
- The vast majority of dust monitors on the marked use a measuring principle based on some variety of reflection light. Light is sent into the process, and then the amount of light reflected by the dust particles or the amount of light absorbed by them is measured. Thus, this direct measurement cannot determine the concentration in mg/m3 since the transmission is dependent on the particle size and colour. As a result, it is necessary to perform a gravimetric/isokinetic measurement and compare it with the signal displayed by the monitor. In this way, the calibration curve on the monitor is determined. It can be said that it converts the monitor's 4-20mA output to 0-xx mg/m3. Typically, this calibration must be performed every 5 years.
- What is important when it comes to instrument air?
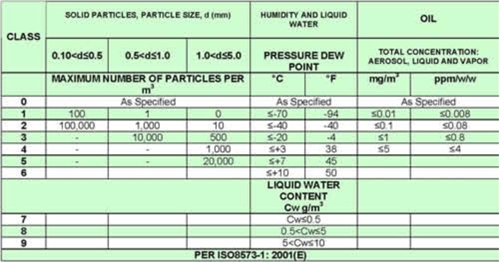
- Instrument air and compressed air must not be compared. Ordinary compressed air which comes directly from a compressor is far from clean. When speaking of instrument air, it is moisture-free, oil-free and particle-free air with a dew point of typically -20°. This entails that the air has passed through a cooling system or a permeation dryer in order to remove moisture. Typically, there will be demands regarding the specific ISO class of the air. Refer to table 1:
Table 1: Instrument air quality according to ISO
 - Installation of CEMS equipment
- CEMS = Continuous Emission Monitoring System. In such systems, the probe must always be placed after the last step of flue gas treatment before the stack or in the stack. This is done to ensure that the measured values reflect the actual emission to the atmosphere. Some components such as CO, O2, NO and CO2 may be measured earlier in the process, provided that they are not influenced by for instance a flue gas condenser or wet scrubber. The probe must be placed on a straight, homogeneous length of the duct, 5 x duct diameter after a bend, restriction, expansion or the like as well as min. 1 x duct diameter before a bend, restriction, expansion or the like.
- Principles and expressions within gas analysis
- What is the difference between NO and NOX?
- Nitrogen oxides typically occur in combustion processes at high temperatures and are often referred to as NO and NO2. Nitrogen oxides released from combustion sources are primarily NO with less occurrence of NO2. NO is colourless and odourless. When ozone is present, NO will form NO2. NO2 is a reddish brown gas, partly responsible for the brown smog seen by some larger cities. In overall terms, NO and NO2 are called NOx (NOx = NO + NO2). In most cases, measurements used for process control will only consist of NO as the NO2 part typically only constitute 5 % of the total NOx. For emission measurements measurement of both NO and NO2 will be required, either as individual measurements or as a measurement of the total NOx in which NO2 is converted to NO and then measured as a sum in the NO analyser.
- NOx conversion:
NOx (ppm) = NO (ppm) + NO2 (ppm)
ppm --> mg/m3:
NO mg/m3 = 1,3387 x NO (ppm)
NO2 (mg/m3) = 2,0525 x NO2 (ppm) - How do I convert from ppm to mg/m3?
- Measurement values may either be specified as ppm or mg/m3 for components that are not specified as Vol%. The factor between these two units varies from component to component. Here are some of the most common: CO 1 ppm = 1.25mg/m3, NO 1 ppm = 1,34 mg/m3, NO2 1ppm = 2.05mg/m3 and SO2 1ppm = 2,86mg/m3. Conversion tables for all components may be found online. Google is very useful.
- Process knowledge
- Why is it important to know the gas composition in the process?
- It is important to know the flue gas composition as cross interference between the different components often exist. By knowing the composition, it is possible to make up for this in the analysis equipment i.a. by using optical filters. The better we know the composition, the better we can design the analyser and avoid unfortunate and costly surprises once the system is running.
- Why is it important to know the pressure in the process?
- Dependent on the measuring equipment to be used, the pressure in the process is a parameter to consider. When it comes to pressure tolerances, every instrument has specific limitations to be complied with in order to use the equipment for the relevant task. In the case of overpressure at the measuring point, it is essential to ensure protection against smudge by means of instrument air or the like. Furthermore, it may be necessary for some equipment to install fail safe shutters in case of instrument air failure. Overpressure at the measuring point also means that special considerations must be taken to avoid injury to the operating staff and service technicians in connection with maintenance and service of equipment. For safety reasons, it is also recommended that the equipment is mounted at a measuring spot with negative pressure.
- Choice of equipment
- Why choose UV rather than IR?
- The majority of continuously measuring gas analysers make use of either UV or IR absorption on the wave length applicable for the individual gas component. In general, it may be said that IR is the cheapest technology, but it has certain limitations. UV is a more expensive technique, but it is more selective; i.e. cross interference from other components in the flue gas does not exist to the same degree as with IR. Some components such as NO2 may only be measured with UV. Components such as NO and SO2 may be measured by either UV or IR. As a rule of thumb, UV will be a necessity for these two components at low measuring ranges (typically below 200 mg/m3) as the cross-interference from water will affect the measurements too much in the IR area.
- What are the advantages and disadvantages of paramagnetic, electrochemical and thermomagnetic oxygen monitors?
- O2 may be measured by various measuring principles
Electrochemical:
Advantages: The most inexpensive measuring principle, not moveable parts, robust construction, not sensitive to contamination (particles, moisture, etc.), no cross-interference and cheap to repair.
Disadvantages: The life span of the cell is typically 2-3 years dependent on the O2 concentration in the process. Less accuracy compared to the paramagnetic measuring principle. Measuring range: 0-25 Vol%.
Thermomagnetic:
Advantages: No moveable parts, robust construction, long life span >10 years, no wear parts, fairly resistant to contamination (particles, moisture, acid, etc.) and the measuring chamber may be cleaned and renovated.
Disadvantages: Relatively long response time (typically 10-20 sec.) compared to electrochemical and paramagnetic measuring principles. May only be used for known types of flue gas due to cross-interference with CO2 (to be cross-compensated against CO2). Expensive in repairs.
Paramagnetic:
Advantages: Quick response time (few sec.), high accuracy compared to electrochemical and thermomagnetic measuring principles. 100% linear 0-100 Vol%. No cross-interference except from high H2 concentrations (Vol%).
Disadvantages: The most expensive measuring principle, sensitive measuring chamber (moveable parts), does not tolerate contamination of particles, moisture or acid. Expensive in repair. - When to use a heated extraction system?
- A heated extraction system (probe and heated hose) must be used to measure components that are easily soluble in moisture; e.g. SO2, TOC, NO2, HCl, NH3 and HF. In these cases, it is important to keep the temperature of the gas sample above dew-point to prevent condensation in the "heated hose". In certain cases, it is also necessary to use a heated analyser if very low emissions values are to be measured.
- What is the difference between NO and NOX?
- Nitrogen oxides typically occur in combustion processes at high temperatures and are often referred to as NO and NO2. Nitrogen oxides released from combustion sources are primarily NO with less occurrence of NO2. NO is colourless and odourless. When ozone is present, NO will form NO2. NO2 is a reddish brown gas, partly responsible for the brown smog seen by some larger cities. In overall terms, NO and NO2 are called NOx (NOx = NO + NO2). In most cases, measurements used for process control will only consist of NO as the NO2 part typically only constitute 5 % of the total NOx. For emission measurements measurement of both NO and NO2 will be required, either as individual measurements or as a measurement of the total NOx in which NO2 is converted to NO and then measured as a sum in the NO analyser.
- NOx conversion:
NOx (ppm) = NO (ppm) + NO2 (ppm)
ppm --> mg/m3:
NO mg/m3 = 1,3387 x NO (ppm)
NOx (mg/m3) = 2,0525 x NOx (ppm)
|

