Home
- Gas Analysis & Reporting
- Dust monitors
- Flow monitors
- Gas analysers
- Oxygen monitors
Condensate pumps- Coolers
- Filters
- Fittings
- Heated hoses
- Hoses
- Probes
- Sample gas pumps
- Test gasses
- Valves
Reconditioned products- Spare parts
- For analysers
- For gas conditioning
- Batteries
- Circuit boards
- Condensate pumps - spare parts
- Coolers - spare parts
- Filters - spare parts
- Fittings - spare parts
- Fuses
- Gaskets
- Heated hoses - spare parts
- Hoses - spare parts
- O-rings
- Other electrical
- Other mechanical
- PLCs
- Power supplies
- Probes - spare parts
- Relays
- Sample gas pumps - spare parts
- Sensors
- Test gasses - spare parts
- Tools
- Valves - spare parts
- Info pages

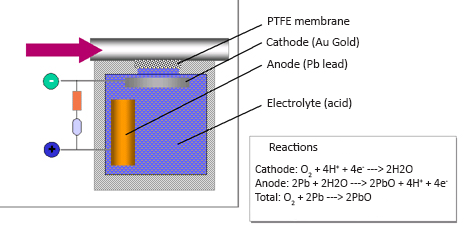 The electrochemical measuring principle is often used for measurement of O2 in extractive gas analysis systems. However, it can also be used for measurement of CO, NO and SO2 from small transportable gas analyser systems. The measurement is made by letting for instance O2 pass through a gas selective membrane, which only allows for the gas to be measured to pass through. The O2-molecules diffuse into an electrolyte solution and are converted to H2O. This reaction is provoked by a gold cathode in the electrolyte. By the anode, which is made of lead, lead oxide is produced by means of the electrons that are released when O2 reacts and is converted to H2O. The migration of electrons between the anode and the cathode represents the amount of O2 converted to H2O, and it is thus an indicator of the O2-concentration in the process.
The electrochemical measuring principle is often used for measurement of O2 in extractive gas analysis systems. However, it can also be used for measurement of CO, NO and SO2 from small transportable gas analyser systems. The measurement is made by letting for instance O2 pass through a gas selective membrane, which only allows for the gas to be measured to pass through. The O2-molecules diffuse into an electrolyte solution and are converted to H2O. This reaction is provoked by a gold cathode in the electrolyte. By the anode, which is made of lead, lead oxide is produced by means of the electrons that are released when O2 reacts and is converted to H2O. The migration of electrons between the anode and the cathode represents the amount of O2 converted to H2O, and it is thus an indicator of the O2-concentration in the process.